A Buffer Solution Must Contain
| BUFFER SOLUTIONS This page describes simple acidic and alkaline buffer solutions and explains how they piece of work. What is a buffer solution? Definition A buffer solution is one which resists changes in pH when minor quantities of an acrid or an alkali are added to it. Acidic buffer solutions An acidic buffer solution is simply one which has a pH less than 7. Acidic buffer solutions are commonly made from a weak acid and one of its salts - ofttimes a sodium salt. A common example would be a mixture of ethanoic acid and sodium ethanoate in solution. In this case, if the solution contained equal tooth concentrations of both the acid and the salt, information technology would have a pH of four.76. It wouldn't matter what the concentrations were, every bit long as they were the aforementioned. Y'all can change the pH of the buffer solution past changing the ratio of acid to salt, or past choosing a dissimilar acid and one of its salts. | |
| Annotation:If you have a very weak acrid and one of its salts, this can produce a buffer solution which is actually alkaline! I will annotate briefly about this further down the page, but if you are doing buffer solutions at an introductory level this isn't likely to bother you lot. If you need to know about calculations involving buffer solutions, you may be interested in my chemistry calculations book. | |
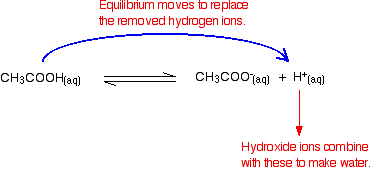
| Alkaline buffer solutions An alkali metal buffer solution has a pH greater than 7. Alkaline metal buffer solutions are commonly made from a weak base and ane of its salts. A frequently used case is a mixture of ammonia solution and ammonium chloride solution. If these were mixed in equal molar proportions, the solution would have a pH of ix.25. Again, it doesn't matter what concentrations you cull as long as they are the aforementioned. How do buffer solutions work? A buffer solution has to contain things which will remove any hydrogen ions or hydroxide ions that you might add to it - otherwise the pH volition change. Acidic and alkali metal buffer solutions achieve this in dissimilar ways. Acidic buffer solutions We'll take a mixture of ethanoic acid and sodium ethanoate as typical. Ethanoic acid is a weak acid, and the position of this equilibrium will be well to the left: Adding sodium ethanoate to this adds lots of extra ethanoate ions. According to Le Chatelier'southward Principle, that will tip the position of the equilibrium even further to the left. | |
| Note:If you don't understand Le Chatelier's Principle, follow this link before you get any farther, and brand sure that you understand about the effect of changes of concentration on the position of equilibrium. Employ the BACK button on your browser to return to this page. | |
| The solution will therefore contain these important things:
Other things (similar h2o and sodium ions) which are present aren't of import to the argument. Adding an acid to this buffer solution The buffer solution must remove nigh of the new hydrogen ions otherwise the pH would driblet markedly. Hydrogen ions combine with the ethanoate ions to make ethanoic acid. Although the reaction is reversible, since the ethanoic acid is a weak acid, nearly of the new hydrogen ions are removed in this fashion. Since most of the new hydrogen ions are removed, the pH won't change very much - merely because of the equilibria involved, information technology will autumn a little flake. Calculation an alkali to this buffer solution Alkali metal solutions contain hydroxide ions and the buffer solution removes most of these. This time the situation is a bit more complicated considering there are two processes which can remove hydroxide ions. Removal past reacting with ethanoic acid The most likely acidic substance which a hydroxide ion is going to collide with is an ethanoic acid molecule. They will react to form ethanoate ions and water. | |
| Note:You might be surprised to find this written as a slightly reversible reaction. Because ethanoic acid is a weak acrid, its conjugate base of operations (the ethanoate ion) is fairly skillful at picking upwards hydrogen ions once more to re-form the acrid. It can get these from the water molecules. You may well find this reaction written as one-style, but to be fussy nigh it, it is really reversible! | |
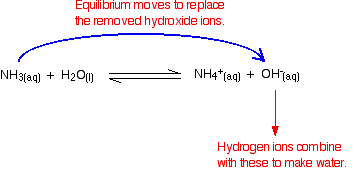
| Because most of the new hydroxide ions are removed, the pH doesn't increase very much. Removal of the hydroxide ions past reacting with hydrogen ions Remember that in that location are some hydrogen ions present from the ionisation of the ethanoic acid. Hydroxide ions tin can combine with these to brand water. As shortly equally this happens, the equilibrium tips to replace them. This keeps on happening until most of the hydroxide ions are removed. Again, because yous have equilibria involved, non all of the hydroxide ions are removed - only nearly of them. The h2o formed re-ionises to a very modest extent to give a few hydrogen ions and hydroxide ions. Alkaline metal buffer solutions We'll take a mixture of ammonia and ammonium chloride solutions as typical. Ammonia is a weak base, and the position of this equilibrium will be well to the left: Adding ammonium chloride to this adds lots of extra ammonium ions. According to Le Chatelier's Principle, that volition tip the position of the equilibrium fifty-fifty further to the left. The solution will therefore incorporate these important things:
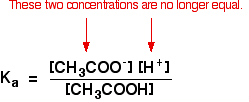
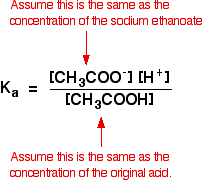
Other things (like water and chloride ions) which are present aren't important to the argument. Calculation an acid to this buffer solution There are two processes which can remove the hydrogen ions that you lot are calculation. Removal by reacting with ammonia The virtually likely bones substance which a hydrogen ion is going to collide with is an ammonia molecule. They will react to form ammonium ions. About, merely not all, of the hydrogen ions will be removed. The ammonium ion is weakly acidic, so some of the hydrogen ions will be released again. Removal of the hydrogen ions by reacting with hydroxide ions Remember that there are some hydroxide ions nowadays from the reaction between the ammonia and the water. Hydrogen ions tin can combine with these hydroxide ions to make water. As presently equally this happens, the equilibrium tips to replace the hydroxide ions. This keeps on happening until most of the hydrogen ions are removed. Again, considering y'all take equilibria involved, not all of the hydrogen ions are removed - just about of them. Adding an brine to this buffer solution The hydroxide ions from the alkali are removed by a simple reaction with ammonium ions. Because the ammonia formed is a weak base of operations, it tin react with the water - and so the reaction is slightly reversible. That means that, over again, most (but non all) of the the hydroxide ions are removed from the solution. Calculations involving buffer solutions This is but a brief introduction. There are more than examples, including several variations, over 10 pages in my chemistry calculations book. Acidic buffer solutions This is easier to run across with a specific case. Remember that an acrid buffer tin be made from a weak acid and 1 of its salts. Allow's suppose that yous had a buffer solution containing 0.10 mol dm-3 of ethanoic acid and 0.xx mol dm-3 of sodium ethanoate. How do you lot calculate its pH? In any solution containing a weak acid, there is an equilibrium between the un-ionised acid and its ions. So for ethanoic acrid, you have the equilibrium: The presence of the ethanoate ions from the sodium ethanoate will have moved the equilibrium to the left, but the equilibrium still exists. That ways that y'all tin can write the equilibrium constant, Ga, for information technology: Where you accept done calculations using this equation previously with a weak acid, yous will accept assumed that the concentrations of the hydrogen ions and ethanoate ions were the same. Every molecule of ethanoic acid that splits up gives one of each sort of ion. That's no longer true for a buffer solution: If the equilibrium has been pushed even further to the left, the number of ethanoate ions coming from the ethanoic acid will be completely negligible compared to those from the sodium ethanoate. We therefore assume that the ethanoate ion concentration is the same every bit the concentration of the sodium ethanoate - in this case, 0.20 mol dm-3. In a weak acrid calculation, nosotros usually assume that and so little of the acid has ionised that the concentration of the acid at equilibrium is the aforementioned as the concentration of the acrid we used. That is even more true now that the equilibrium has been moved fifty-fifty farther to the left. So the assumptions nosotros make for a buffer solution are: Now, if we know the value for Ka, nosotros can calculate the hydrogen ion concentration and therefore the pH. Ka for ethanoic acid is 1.74 x 10-five mol dm-iii. Recollect that we desire to summate the pH of a buffer solution containing 0.ten mol dm-3 of ethanoic acid and 0.20 mol dm-3 of sodium ethanoate. Then all y'all have to practice is to discover the pH using the expression You will yet take the value for the hydrogen ion concentration on your calculator, then press the log push button and ignore the negative sign (to allow for the minus sign in the pH expression). You should get an respond of 5.1 to ii significant figures. You can't exist more accurate than this, because your concentrations were just given to two figures. | |
| Note:I commented further upward the page that if yous had a very weak acid and i of its salts, the buffer solution formed could well be alkaline. An instance is a mixture of HCN and NaCN. HCN is a very weak acid with a Ka of iv.9 x ten-10 mol dm-3. If you had a solution containing an equal numbers of moles of HCN and NaCN, you lot could summate (exactly every bit in a higher place) that this buffer solution would have a pH of 9.iii. This isn't something that you need to worry about. Just don't assume that every combination of weak acid and i of its salts will necessarily produce a buffer solution with a pH less than 7. | |
| You could, of course, be asked to reverse this and summate in what proportions you would have to mix ethanoic acid and sodium ethanoate to go a buffer solution of some desired pH. It is no more difficult than the calculation we have just looked at. Suppose you wanted a buffer with a pH of 4.46. If you un-log this to find the hydrogen ion concentration yous need, you lot will find it is three.47 x 10-5 mol dm-3. Feed that into the Chiliada expression. All this means is that to get a solution of pH 4.46, the concentration of the ethanoate ions (from the sodium ethanoate) in the solution has to be 0.5 times that of the concentration of the acid. All that matters is that ratio. In other words, the concentration of the ethanoate has to be one-half that of the ethanoic acid. Ane manner of getting this, for case, would be to mix together x cmiii of 1.0 mol dm-3 sodium ethanoate solution with 20 cmthree of 1.0 mol dm-three ethanoic acid. Or 10 cm3 of one.0 mol dm-iii sodium ethanoate solution with 10 cm3 of two.0 mol dm-3 ethanoic acid. And at that place are all sorts of other possibilities. | |
| Notation:If your maths isn't very good, these examples can await a bit scary, merely in fact they aren't. Become through the calculations line by line, and make sure that you can see exactly what is happening in each line - where the numbers are coming from, and why they are where they are. Then go away and practise like questions. If yous are good at maths and tin can't see why anyone should think this is difficult, then experience very fortunate. Most people aren't and so lucky! | |
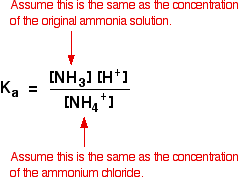
| Alkaline buffer solutions We are talking here about a mixture of a weak base and one of its salts - for instance, a solution containing ammonia and ammonium chloride. The modern, and like shooting fish in a barrel, way of doing these calculations is to re-think them from the point of view of the ammonium ion rather than of the ammonia solution. Once you have taken this slightly different view-point, everything becomes much the same as before. And so how would you find the pH of a solution containing 0.100 mol dm-3 of ammonia and 0.0500 mol dm-3 of ammonium chloride? The mixture will contain lots of unreacted ammonia molecules and lots of ammonium ions as the essential ingredients. The ammonium ions are weakly acidic, and this equilibrium is set whenever they are in solution in water: Yous tin can write a Ma expression for the ammonium ion, and make the aforementioned sort of assumptions as we did in the previous case: The presence of the ammonia in the mixture forces the equilibrium far to the left. That means that you can assume that the ammonium ion concentration is what you started off with in the ammonium chloride, and that the ammonia concentration is all due to the added ammonia solution. The value for Ka for the ammonium ion is v.62 x 10-10 mol dm-3. Remember that nosotros want to calculate the pH of a buffer solution containing 0.100 mol dm-3 of ammonia and 0.0500 mol dm-3 of ammonium chloride. But put all these numbers in the Ka expression, and exercise the sum:
To the acid-base equilibria menu . . . To the Physical Chemistry menu . . . To Main Carte . . . © Jim Clark 2002 (last modified Jan 2016) | |
A Buffer Solution Must Contain,
Source: https://www.chemguide.uk/physical/acidbaseeqia/buffers.html
Posted by: dupreanducce.blogspot.com

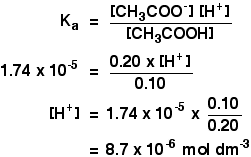
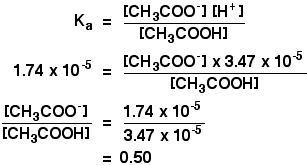
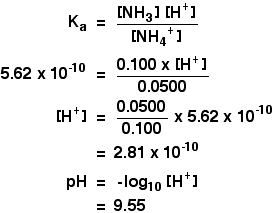

0 Response to "A Buffer Solution Must Contain"
Post a Comment